The U.S. Meals and Drug Administration is telling folks to not use chemical peels at house, warning that the merchandise could cause chemical burns and different critical issues. The company has additionally begun going after sellers of those merchandise, comparable to Amazon and Walmart.
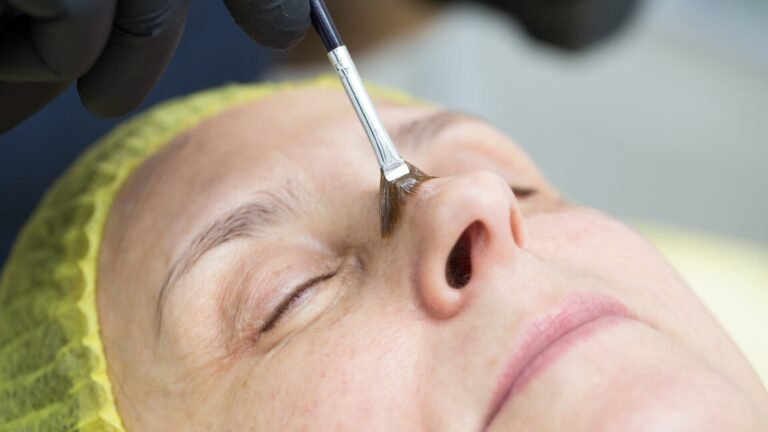
Chemical peels have lengthy been used Dermatologists and beauty surgeons to enhance one’s look. These chemical substances take away broken pores and skin cells from the outer (and generally center) layers of pores and skin, leaving pores and skin that’s typically smoother and fewer wrinkled. Peels are used repeatedly to deal with zits scars, wrinkles, sun-damaged pores and skin, and hyperpigmentation.
Though they’re invaluable, these peels must be carried out beneath the supervision of a skilled skilled. in its warning launch On Tuesday, the FDA famous that it has not but authorised any chemical peels. Attempting to make use of these merchandise your self could also be riskier than ordinary.
“[Chemical peels] Comprise various concentrations of components comparable to trichloroacetic acid (TCA), glycolic acid, salicylic acid, and lactic acid which can be too excessive to be secure at house with out the supervision of a dermatologist or different licensed and skilled practitioner use,” the FDA wrote within the warning.
The company has acquired experiences of opposed occasions, some critical, in individuals who might have been injured by these merchandise. If used incorrectly, chemical peels could cause not solely burns, but in addition ache, swelling, an infection, pores and skin colour modifications, and disfiguring scars. “These accidents might even require emergency or specialty care from a dermatologist or surgeon,” the FDA stated.
Along with the general public warning, the FDA despatched warning letters to 6 corporations that promote these merchandise, together with Amazon and Walmart. The FDA says that as a result of at-home chemical peels haven’t but been authorised, the advertising and marketing of such merchandise is illegitimate. “Failure to adequately deal with this concern might end in authorized motion, together with however not restricted to seizure and/or injunction,” the FDA wrote in a letter to the businesses.
The company can also be asking sufferers and physicians to report opposed occasions associated to those merchandise.